
姓名: 林佳奇
职称: 教授 博士生导师 硕士生导师
性别: 男
毕业院校:大连理工大学
学位: 博士
在职信息: 在职
所在单位: 生物工程学院
学科: 生物工程
办公地点: 大连理工大学生物工程学院513
联系方式: 0411-84706184
电子邮箱: jqlin@dlut.edu.cn
课题组针对人体各种生物屏障的机理性研究,设计、开发和制备克服各种生物屏障的生物医学材料。聚焦的生物屏障包括血液循环系统屏障、组织间隙屏障、细胞膜屏障、内涵体屏障、胃肠道系统屏障、皮肤屏障等。主要研究方向:
1) 核酸药物递送。包括多种ASO、siRNA、mRNA药物和疫苗制剂的设计和制备;通过对药物和多层生物屏障相互作用的研究,理性设计药物载体和给药方式,提高核酸药物的递送效率并减少副作用。
2) 纳米生物界面。通过纳米材料与生物屏障的相互作用的研究,建立纳米生物界面的基本作用原理。研究不同物理化学性质的纳米材料克服不同生物屏障的能力,如穿膜和逃逸内涵体的能力,以及纳米材料所产生的生物效应。
3) 人机交互。通过皮肤与生物材料接触界面的研究,设计可长时间采集高质量的人体生物电信号的生物材料,应用于脑机接口、智能假肢、智能手环以及VR/AR等多种可穿戴设备的信号采集。
团队负责人林佳奇博士曾分别在美国麻省理工学院材料科学与工程系和美国麻省理工学院科赫癌症研究中心做博士后研究员。期间,林佳奇博士在纳米生物界面领域,尤其是药物递送系统在体内环境输运以及药物释放等动力学行为和机制取得了突破的进展。迄今为止在著名学术期刊Nature Biotechnology, Nature Biomedical Engineering, Nature communications, Nano Letters, ACS Nano, Science Advances, Small, Communications Biology等期刊上发表论文20余篇,2篇论文入选ESI高被引论文,他引1200余次,h-index=12。研究成果可化为新型核酸疫苗应用于新冠等传染病的预防,其中包括新冠疫苗、流感疫苗等、HPV疫苗等,目前已经跟科兴疫苗展开合作。
此外,负责人林佳奇博士开发了半干水凝胶柔性电极,成功解决了当前脑电/肌电电极的信号质量差以及难以重复使用的问题。团队完成了该电极从实验室原型到量产的过程(包括有机合成、调配、成型、装配的标准化等),实现了该电极的量产。目前电极产品销售于15个国家和地区,脑电产品受到了美国宇航局NASA的报道,应用于美国奥林匹克运动队;肌电产品被美国《时代》杂志评为2019年度100个最佳发明之一。
The Lab has been engaged in the field of nano-biological interfaces, and the results mainly focus on the interaction of nanomaterials and cell membranes, the cellular entry pathway of nano-carriers, the endosomal escape mechanism of nanomaterials, the aggregation and protein adsorption of nanomaterials, the targeted drug delivery system for oral administration, the molecular mechanism of nanopore formation in cell membranes, the interaction between conductive gel and skin interface, etc. Relevant results were published as the first author in many well-known academic journals, two of which are ESI high-citing articles, with a total of more than 1,200 citations, h-index=12. The Lab’s research revolves around a variety of biological barriers, including endosomal barriers, cell membrane barriers, blood circulation clearance barriers, gastrointestinal mucosal barriers, and skin barriers. We intend to conduct mechanistic research on each biological barrier, expounding the mechanism of action between nanomaterials and biological barriers, so as to guide the design and preparation of nano-biological materials that overcome specific biological barriers. At the same time, explore the practical application of each material that overcomes the biological barrier, and combine the industrialization platform of schools and enterprises to develop products that solve practical problems.
克服生物屏障材料的设计以及其在药物递送和人机交互领域的应用。

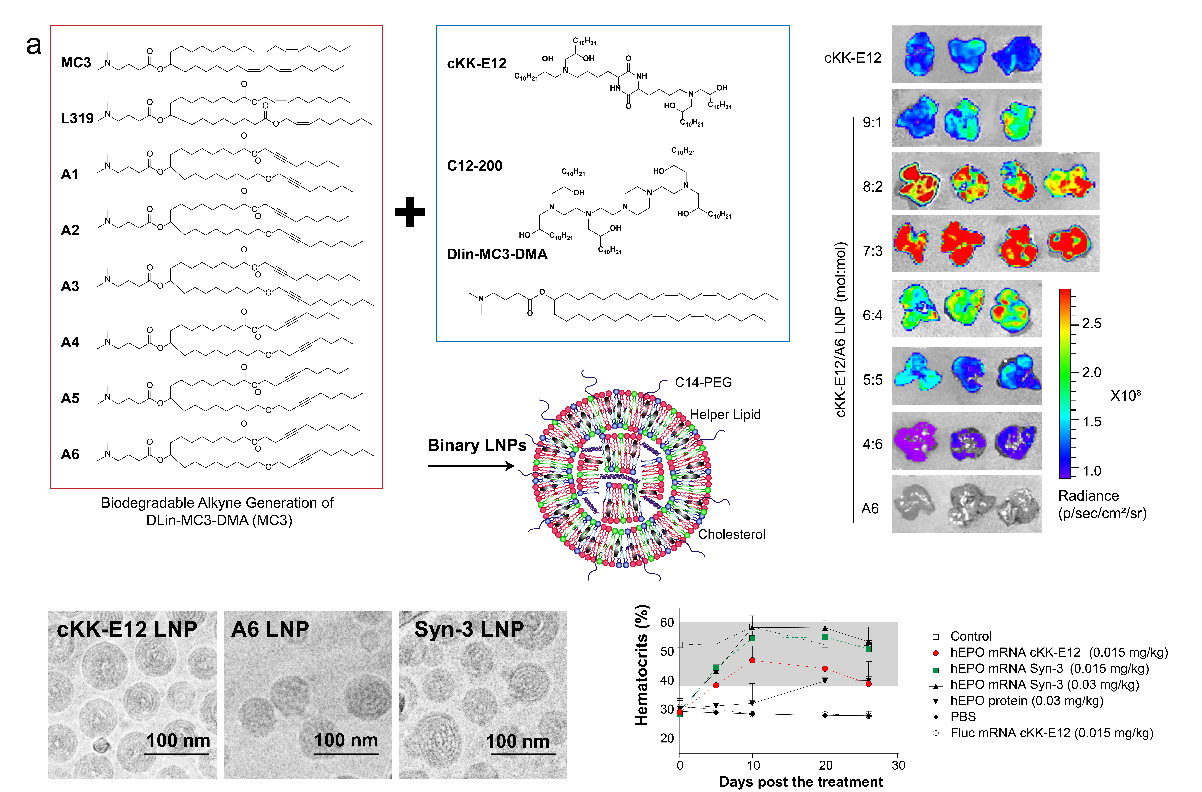
复合型脂质基因载体。体内表达mRNA的效果高于现阶段商业化的脂质载体,并且具有很好的耐受性。
半干水凝胶柔性电极。该新型电极可长时采集高质量生物电信号,应用在注意力检测(脑电)和智能假肢(肌电)上。

【1】林佳奇等,mRNA疫苗递送载体及制备方法、mRNA疫苗及制备方法,申请公布号:CN 111658782 A
【2】林佳奇等,一种mRNA转染材料、mRNA转染系统及应用,申请公布号:CN 114540423 A
【3】林佳奇等,一种响应型核酸递送系统及其制备方法、交联聚合物载体,申请公布号:CN 114984236 A
【4】林佳奇等,一种利用核仁素增强mRNA稳定表达的方法,申请公布号:CN 113817778 A
【5】林佳奇等,一种可用于脑电信号传感器且生物相容的导电水凝胶的制备,申请公布号:CN 113817180 A
1. Lin, J.; Lei, M.; Zhong, G.; Lin, C.; Dargazangy, R.; Alexander-katz, A. Understanding the Synergistic Effect of Physicochemical Properties of Nanoparticles and their Cellular Entry Pathways. Communications Biology, 2020, 3, 205
2. Miao, L.*; Lin, J.*(co-first); Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D. Synergistic Lipid Compositions for Albumin Receptor Mediated Delivery of mRNA to the Liver. Nature communications, 2020, 11, 2424
3. Von Erlach, T.; Saxton, S.; Shi, Y.;Minahan, D.; Reker, D.; Javid, F.; Lee, Y.; Schoellhammer, C.; Esfandiary, T.; Cleveland, C.; Booth, L.; Lin, J.; Levy, H.; Blackburn, S.; Hayward, A.; Langer, R.; Traverso, G. Whole tissue robotic interface system for oral drug formulation development. Nature Biomedical Engineering, 2020, 4, 544–559.
4. Miao, L.; Li, L.; Huang, Y.;Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; Doloff, J.; Langer, R.; Anderson, D. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nature Biotechnology, 2019, 37, 1174-1185
5. Wang, D.; Lin, J.; Jia, F.; Shimkonis, J. C.; Nyoni, Z.; Lu, X. and Zhang, K. Bottlebrush-architechtured Poly(ethylene Glycol) as an Efficient Vector for RNA Interference in Vivo. Science Advance , 2019, 5, eaav9322
6. Tang, Y.*; Wu, S.*; Lin, J.*(co-first); Cheng, L.; Zhou, J. Liao, G. and Li, C. Nanoparticles Targeted against Cryptococcal Pneumonia by Interactions between Chitosan and Its Peptide Ligand. Nano Letters, 2018, 18, 6207-6213
7. Liu, J; Pang, Y.; Zhang, S.; Cleveland, C.; Yin, X.; Booth, L.; Lin, J.; Lee, Y-A.; Mazdiyasni, H.; Saxton, S.; Kirtane, A.; von Erlach, T.; Rogner, J.; Langer, R. and Traverso, G. Triggerable tough hydrogels for gastric resident dosage forms. Nature Communication , 2017, 8:124
8. Lin, J.; Morovati, V.; Zhang, H.; Dargazany, R. PEGylation on mixed monolayer gold nanoparticles: Effect of grafting density, chain length, and surface curvature. Journal of Colloid Interface and Science, 2017, 504, 325-333
9. Lin, J.; Dargazany, R.; Alexander-Katz, A. Lipid flip-flop and pore nucleation on zwitterionic bilayers are asymmetric under Ionic Imbalance. Small, 2017, 13, 1603708
10. Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; Lee, H.; Iverson, N.; Bisker, G.; Li, L.; Strano, M.; Weitz, D. and Anderson, D. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Letters, 2017, 17, 2015-2020
11. Dargazany, R.; Chen, H.; Lin, J.; Itskov, M; Alexander-Katz, A. On the validity of representation of the inter-particle forces of a polymer-colloid cluster by linear springs. Polymer, 2016, 109, 266-277
12. Dargazany, R.; Lin, J.; Khalili, L.; Itskov, M; Chen, H.; Alexander-Katz, A. Micromechanical model for isolated polymer-colloid clusters under tension. Physical Review E, 2016, 94, 042501
13. Lin, J.; Alexander-Katz, A. Probing Lipid Bilayer under Ionic Imbalance. Biophys. J, 2016, 111, 2460-2469.
14. Lee, Y.A.; Zhang, S.; Lin, J.; Langer, R.; Traverso, G. A Janus Mucoadhesive and Omniphobic Device for Gastrointestinal Retention. Adv. Healthc. Mater , 2016, 5, 1141-1146.
15. Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano, 2013, 7, 10799-10808.
16. Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. A Simulation Study of Aggregations of Monolayer-Protected Gold Nanoparticles in Solvents. J. Phys. Chem. C, 2011, 115, 18991–18998.
17. Lin, J.; Zheng, Y.; Zhang, H.; Chen, Z. A Simulation Study on Nanoscale Holes Generated by Gold Nanoparticles on Negative Lipid Bilayers. Langmuir, 2011,27, 8323–8332.
18. Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity and Their Relationship. ACS Nano, 2010,4, 5421-5429.
19. Xie Yusheng#; Du Shubo#; Liu Zhiyang; Liu Min; Xu Zhiqiang; Wang Xiaojie; Kee Jia Xuan; Yi Fan; Sun Hongyan; Yao Shao Q.; Chemical Biology Tools for Protein Lysine Acylation, Angew. Chem. Int. Ed., 2022, 134, e202200303(#共同一作)
20. Du Shubo; Liew Si Si; Zhang Cheng-wu; Du Wei; Lang Wenjie; Yao Cassandra C. Y.; Li Lin; Ge Jingyan*; Yao Shao Q.*; Cell-Permeant Bioadaptors for Cytosolic Delivery of Native Antibodies: A “Mix-and-Go” Approach, ACS Central Science , 2020, 6, 2362-2376.
21. Du Shubo; Liew Sisi; Li Lin*; Yao Shao Q.*; Bypassing Endocytosis: Direct Cytosolic Delivery of Proteins, Journal of the American Chemical Society, 2018, 140, 15986-15996.
22. Du Shubo; Wang, Danyang; Lee, Jun-Seok; Peng, Bo; Ge, Jingyan; Yao, Shao Q.; Cell type selective imaging and profiling of newly synthesized proteomes by using puromycin analogues, Chemical Communications, 2017, 53, 8443-8446.
23. Qu Yunwei; Zhan Qing;Du Shubo*; Ding Yang; Fang Bin; Du Wei; Wu Qiong*; Yu Haidong; Li Lin*; Huang Wei; Catalysis-based specific detection and inhibition of tyrosinase and their application, Journal of Pharmaceutical Analysis, 2020, 10(5): 414-425.
林佳奇 博士 教授
Prof. Jiaqi Lin
Tel: 0411-84706184
E-mail: Jqlin@dlut.edu.cn
都书博 博士 副教授
Prof. Shubo Du
E-mail: dushubo@dlut.edu.cn
刘霖 博士 工程师
Dr. Lin Liu
Tel: 0411-84708850